|
NO ME SALEN
(APUNTES TEÓRICOS DE BIOFÍSICA DEL CBC)
SEGUNDO PRINCIPIO DE LA TERMODINÁMICA
|
|

|
| |
The Timeline
The Second Law of Thermodynamics (or 2nd Law) is my favorite law, the most beautiful of the laws of the universe that we (humans) have manage to unravel.
The reason for my preference is that the rest of the laws represent, ultimately, a balance which does not distinguish between past and future. Instead the 2nd Law Is the only one that tells us that the present is not equal as the past, and therefore, that the future will not be the same as the present.
That exquisite particularity of the 2nd Law made many physicists and philosophers call it ... The Timeline. Just look at the kind of stuff it deals with:
- A vase is likely to fall to the ground and shatter; instead it is unlikely that a set of pieces will spontaneously assemble and make up a vase.
- Gas molecules move completely randomly, but it is almost impossible to find in a room (for example) all the nitrogen molecules on one side and the oxygen molecules on the other... we always find them homogeneously mixed.
- It is relatively easy to drive a double trailer truck. It is almost impossible to go reverse with it.
- Perpetual motion machines can not exist.
- Although energy is conserved ... it degrades.
Another unusual thing about the 2nd Law is that it has innumerable possible statements. There are like a hundred different definitions dispersed in the physics texts. And over twenty of them are famous and bear the author's name. Here are some: |
| |
| |
Kelvin definition: It is not possible a process whose only result is the absorption of heat coming from a focus and the conversion of this heat into work. |
|
(1) |
|
|
|
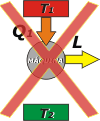 |
What Lord Kelvin (William Thompson, 1824-1907) tells you, is that in a certain way it is impossible for a thermal machine to extract heat from the boiler and transform it entirely into work. It is not about the difficulty of developing a machine like that... the thing is that the structure of the universe wouldn't allow such machine to exist. |
|
|
 |
| |
Clausius definition: No process is possible whose only result is the extraction of heat from a vessel at a certain temperature and the absorption of an equal amount of heat by a vessel at a higher temperature. |
|
(2) |
|
|
|
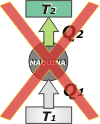 |
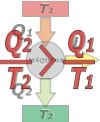
What Rudolf Emanuel Clausius (1822-1888) tells you in some sort of way, is that it cannot exist a refrigerating machine capable of extracting heat from a "cold source" and deliver it to a "hot source" without doing a work on the machine. Again, It is not about the difficulty of developing a machine like that... |
|
|
 |
A salient characteristic of the different statements of the 2nd Law is that all of them seem to talk about different things.However, they are all about the same thing. In fact, there are theorems that show the equivalence between one definition and another. The most famous of these theorems the one which demonstrates the equivalence between the formulations of Clausius and Kelvin.
Read this other statement: |
|
|
| |
Divine statement: there is a state function (called entropy) such that in a real process always increases. Psalms, 7:15 |
|
(3) |
|
|
Here there is an article about entropy.
|
 |
What this statement is telling you is that if you sum the entropy variations of all phenomena associated with any process, the sum is always greater than zero. For example, this is the case of a thermal machine: the entropy change of the boiler (which is negative), plus the entropy change of the environment (which is positive), gives a number greater than zero. You will realize that this divine definition ... allows you to make equations and calculations. |
|
|
 |
A variant of the last one... |
|
|
| |
Universal statement: in any process, the entropy of the universe increases. |
|
(4) |
|
|
|
Watch it carefully... the entropy of anything can diminish. For example, if you put a glass of water in the refrigerator, the water freezes and its entropy decreases. But for that to happen, a refrigerating machine must necessarily be running, and there must be an electric consumption and there must be a radiator taking out the heat to the back of the refrigerator... And if you sum all the entropy changes that had to occur in order to get your glass cool ... there's no doubt about it: total entropy will have increased.
Don't go yet. I have more definitions. |
|
|
| |
Analogical statement: Entropy can not be destroyed, but it can be created. |
|
(5) |
|
|
|
That one was made in a strong analogy with the First Principle. Energy was not created or destroyed ... instead, entropy is being created permanently. Ingenious, right?
Check out this one: |
|
|
| |
"Ring-beller" definition: the universe always evolves to a more probable state. |
|
(6) |
|
|
|
 |
What this statement is telling you is that the universe by itself adopts states more likely to be found statistically.
If you roll six dice, the probability of getting six equal numbers is pretty low. Well, in any spontaneous process the universe moves from a less probable state to a more probable state. |
|
|
|
If every object in your room would have a random place, your mom would kill you. But the thing is that by itself, the room gets disordered every moment, and always ends up being a chaos.
The following definition is related. |
|
|
| |
Beach statement: every system moves to a state of greater equilibrium. |
|
(7) |
|
|
|
Which explains why the sand castles disappear from the beach landscape and evolves into the smooth undulations of the floor.
Another one, an that's all folks: |
|
|
| |
Fateful statement: the universe moves inexorably towards a deplorable state of thermal equilibrium. |
|
(8) |
|
|
|
And that will be the end, because as you well know, thermal equilibrium is nothing other than an equal and uniform temperature for all bodies, that is, the impossibility of heat exchange ... glup.
Believe it or not, all those definitions are equivalent, they all point out to the same thing (but expressed from different angles). |
|
|
| |
|
|
Curious Facts |
|
|
- According to Lynn Margulis and Dorion Sagan, nature invented sex to accelerate the process of energy degradation, that is, to accelerate the increase in entropy (What a materialistic people!).
- Sometimes a Third Law of Thermodynamics is mentioned. Its author, Walther Nerst (1864-1941) put it this way: entropy tends to zero at the zero absolute temperature.
|
|
 |
Captious Questions |
|
- Could the kingdom of heaven be real?
|
|
|
|
|
| Some rights reserved. Reproduction permitted if quoting the source. Last updated on Jan-17.Translated by Esteban Djeordjian. Buenos Aires, Argentina. |
|
|
 |
| | |
|
|