|
NO ME SALEN
(APUNTES TEÓRICOS DE BIOFÍSICA DEL CBC)
PRIMER PRINCIPIO DE LA TERMODINÁMICA
|
|

|
| |
First Law of thermodynamics
The First Law is nothing but the way the general Principle of Energy Conservation fits here, in the thermodynamics' neighborhood. However, because the First Law was the original formulation, some physicists claim for it a special pre-eminence. Let them fight: now we don't care about that.
The general Principle of Conservation of Energy says something like |
| |
| |
Energy is not created or destroyed, it only transforms. |
(1) |
|
|
|
In order to understand this Principle in thermodynamics, we'll go back in time to the Industrial Revolution and try to describe what happens to an newly invented machine when it is started up and it gets to work. |
|
|
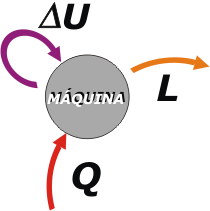 |
What comes next is important, since it acts as a reference system: let's focus on the machine's point of view.
It turns out that the machine needs to receive a certain amount of heat, Q, in order to work, , which usually comes from a boiler, a combustion chamber or any other place where you can burn fuel bought in a gas station, or elsewhere.
What does the machine do with that energy in the form of heat? Part of that energy it's used by the machine to heat itself. This is a bit useless, unwanted (no one likes the heat raise in their machines), but this cannot be avoided |
|
|
|
Machine's heat is stored (at least temporarily) as an internal energy, U (actually as an increase of its internal energy, ΔU, since we cannot pretend that before it was zero), that in this case depends exclusively on the temperature.
Later, we’ll see that other bodies -more sophisticated than an iron machine - can store internal energy in other ways. And the most interesting part of what the machine does with the energy we give it is: work, L, do something we want (moving the wheels of a locomotive, the propeller of a ship, warps of a loom, etc.). L and W are symbols accepted for work and will use them interchangeably.
Let's review: Q is the heat delivered to the machine. ΔU is the internal energy variation of the machine. And L is the work that the machine provides. With First Law of thermodynamics, we can assure that : |
|
|
|
|
|
But machines also lose heat (everybody knows that near working machines things get hot, that they shouldn't be touched in order to avoid getting burn, and that they throw fire jets through leaks or chimneys, or by wherever they can, depending on what they do) |
|
|
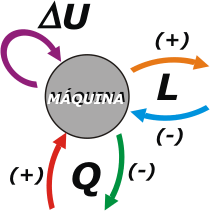 |
To the heat received by the machine, we will assign positive sign. To the heat dismissed by the machine, a negative sign.
To split hairs (later, this precision will be a need) it could also happen that instead of the machine doing a work on the surrounding environment, the environment itself could be doing a work on the machine. This would be a negative work.
These last two are conventions of signs (we could have put them in reverse, but tradition is stronger). The internal energy variation does not require convention of signs since it is, precisely, a variation between two states. When subtracting the final state minus the initial one, it will be positive if there is an increase, and it will give negative if there is a decrease |
|
|
The criterion for signs' convention is the one adopted by the IUPAC
|
The variation of internal energy - in a machine - is only appreciable when the machine starts (for example, in the early morning) or when it is turned off. But at constant rate, always working at the same temperature, during the entire production day, the variation of internal energy is zero. Remember that we are talking about a machine, a simple object, that its only way to accumulate internal energy is with temperature (with the agitation of its molecules). Same happens with ideal gases |
|
|
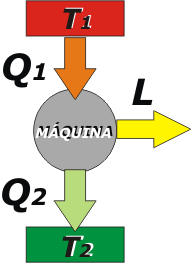 |
Then, if we have a machine operating at constant rate, this is usually represented by a scheme like the one at the left, in which ΔU (the variation of internal energy) does not appear, and in which the heat source (the boiler or whatever) is shown separated from the sink source (the environment receiving the wasted heat).
In thermodynamics we call source to a body that gives or receives heat and does not change its temperature. This is the case of a well-managed boiler: if the stoker avoids firewood' shortage, the boiler will transfer its heat, Q1, without lowering his temperature, T1. This goes for any combustion chamber.
And it is also the case of the wasted heat sink, Q2, which receives the heat without increasing the temperature, T2, - in this case - because it is the environment, which is immense compared to the machine. |
|
|
|
If we describe this super important description (because it is exportable to many cases, not just machines) with the First Law, it would look like: |
|
|
| |
Q1 – Q2 = L |
Constant rate: (ΔU = 0) |
|
|
|
If you made it until here, make a last effort and read the following facts. |
|
|
Curious Facts |
|
|
- For some reason most textbooks present the First Law in this way: ΔU = Q - L, which is equivalent to the presented equation. I recommend you to use "my" equation and not the one from the books, reserving the sign assignment for the convention energy received / energy delivered (many students screw it up when faced with a double assignment of signs). In addition, if you want to write down the First Law by heart, my formulation is simple to remember: the heat received is used for this and for that, a = b + c. In contrast with the other formulation ... what was it like? Q-L or L-Q ...? Well ... what where you expecting ... I'm kinda fool.
- First Law of thermodynamics, as shown by the spirit of this article, was intended to describe the operation of machines and engines that emerged during the Industrial Revolution. But later it was found to be fruitfully descriptive for any system: simple, complex, inert, or even alive!
- When it comes to a living organism the main contribution of energy, is the chemical energy that comes with food ... which becomes an increase of the internal energy, ΔU. A very particular mechanism, called hunger, tells us that we must increase our internal energy in order to continue doing jobs or losing heat ... that is, to continue living.
- Francis Bacon, Robert Hooke, and Isaac Newton said in the seventeenth century that heat was a property of the heated body and resulted from the vibratory movement or agitation of its parts. They were really close.
- The first formulation of the First Law is due to Saadi Carnot and dates from 1850. The relationship between heat and work was already established thanks to the works of James Prescott Joule, William Thomsom (Lord Kelvin), Rudolf Clausius, and others. Carnot added the issue about the internal energy variation, and with that, the First Law was ready.
|
|
|
Captious Questions |
|
|
- Could it be that there is a Zero Law of thermodynamics, and that I have missed it?
|
|
|
|
|
|
(1) Actually the energy itself can vary its total amount, since it can become matter, and vice versa, matter can become energy (In other words, it is actually created and it is actually destroyed). This is an important discovery made by Einstein which is part of Special Relativity Theory, which includes the famous equation of equivalence between matter and energy: E = mc² (Where E is energy, m is mass and c is the speed of light). But both the matter conservation principle and the energy conservation principle are expressed with implicit excluding of the occurrence of relativistic phenomena, which are restricted to atomic bombs, nuclear power plants, star nuclei, and some subatomic interactions.
But on classical mechanics (that is, this course of Physics) we can obviate all relativistic effects and validate the principle of conservation of energy as I stated above. |
|
|
 |
| |
| |
|
|
| Some rights reserved. Reproduction permitted if quoting the source. Last updated on Dec-16.Translated by Esteban Djeordjian. Buenos Aires, Argentina. |
|
|
 |
| | |
|
|