|
NO ME SALEN
(THEORETICAL NOTES OF BIOPHYSICS)
FLUIDS
DENSITY and SPECIFIC WEIGHT |
|
|

|
One kilo of water occupies a volume of a liter. Half a kilo occupies half a liter, and the volume occupied by 653.4 liters equals exactly 635.4 liters. Apparently, the relation between mass and volume is a constant.
But lets choose another totally different liquid, let's say mercury (which, despite being a metal, is a liquid). In a liter bottle you can get 13.6 kilograms of mercury. If the bottle had two liters, you’d get 27.2 kilograms and if you had a little bottle of just 0.1 liters, only 1.36 kilograms would fit. As you see, the quotient between mass and volume, also here, remains constant.
This is how we can express it for any liquid: |
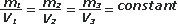
|
|
|
This property is called density and its defined as the quotient between de mass and the volume of any given amount of matter. In the case of water, it's worth 1 kg/l. The symbol for density is the greek letter rho, ρ. |
|
|
|
|
Sometimes
I'll use Vol instead of V, for clarity. |
Each and every substance usually haves a characteristic density. This is an intrinsic property since it does not depend on the amount of what amount of that substance we study. Densities can be measured in any mass unit over any volume unit. The “official” ones would be: |
|
|
| |
|
|
And all of this below, all very common, are equal amongst them and 1,000 times bigger than the “official”:
kg/l = kg/dm3 = g/ml = g/cm3 = mg/μl = mg/mm3
If instead of considering the mass, we consider weight, we can define a new magnitude, specific weight, which is similar to density. It's symbol is the greek letter gamma, γ. |
|
|
|
|
|
If we also remember that the weight of any given body closet to earth surface is:
P = m . g
We can establish a relationship between density and specific weight:
γ = ρ . g
In the following chart we'll find some substances of biological relevante and their densities and specific weight. As you can see, there are not only liquids. |
|
|
|
densidty (ρ) |
especific weight (γ) |
|
kg/l |
kg/m3 |
kgf/l |
kgf/m3 |
N/m3 |
Water (4°C) |
1 |
1,000 |
1 |
1,000 |
10,000 |
Seawater (15°C) |
1.025 |
1,025 |
1.025 |
1,025 |
10,250 |
Ice |
0.917 |
917 |
0.917 |
917 |
9,170 |
Human blood (37°C) |
1.06 |
1,060 |
1.06 |
1,060 |
10,600 |
Blood plasma (37°C) |
1.027 |
1,027 |
1.027 |
1,027 |
10,270 |
Ethyl alcohol |
0.8 |
800 |
0.8 |
800 |
8,000 |
Olive oil |
0.92 |
920 |
0.92 |
920 |
9,200 |
Mercury (20°C) |
13.6 |
13,600 |
13.6 |
13,600 |
136,000 |
Cold air (0°C, 1 atm) |
0.00129 |
1.29 |
0.00129 |
1.29 |
12.9 |
Hot air (100°C, 1 atm) |
0.00095 |
0.95 |
0.00095 |
0.95 |
9.5 |
Earth Planet |
5.17 |
5,170 |
- |
- |
- |
Balsa wood |
0.12 |
120 |
0.12 |
120 |
1,200 |
Common wood |
0.7 |
700 |
0.7 |
700 |
7,000 |
Iron |
7,8 |
7,800 |
7.8 |
7,800 |
78,000 |
| Plumbum |
11.4 |
11,400 |
11.4 |
11,400 |
114,000 |
Gold |
19.3 |
19,300 |
19.3 |
19,300 |
193,000 |
| Notes: in this chart is used g = 10 m/s². To correct te values of specific weight must be multiplied by 0.98. The units and messures in bold belong to the International Sistem (SI) |
|
|
COMMENTS: look in the chart for the only two gases, hot and cold air. They have a density of approximately a thousand times lesser than the rest. In many substances you'll find a temperature indicator; that's because almost every substance expands as it increases its temperature, increasing its volume as well. In liquids and solids, this variation is too small to be considered, but not with gases. When it comes to gases, both temperature and pressure make huge differences.
|
|
|
ATTENTION
In latin literature δ (delta) is used for density, and ρ (rho) for specific weight. |
|
|
| IMPORTANT GOSSIP: |
|
|
- The approximate density of the human body is 0,95 kg/dm3; that's why we float so easily on water.
- The floating issue depends on densities, I explain that in the chapter about Archimedes.
- Many people mistake density with viscosity. Stay alert.
|
|
|
| TRICKY QUESTION: |
|
 |
- Can you explain the physics behind floating on your back?
- The fact that water density is worth 1, is a coincidence?
- Why is it that Earth doesn't have a specific weight?
|
|
Remember that the fractional expressions (both, operations as the units) must be written with the horizontal division line. In many parts of this site I write it oblique and the denominator in the same line, that is not correct, but the html does not allow it if it is not inserting images. |
|
|
 |
|
| Translated by Matías Varela. Some rights reserved.
Be copied by quoting the source. Last updated
sep-07. Buenos Aires, Argentina. |
|
|
| | |
|
|