NO ME SALEN
(LECCIONES TEORICAS DE BIOFÍSICA DEL CBC)
FLUIDS
TRANSPORTATION MECHANISMS – DIFFUSION
|
|

|
| |
Our inside is exchanging molecules of all kinds, all the time. One of the primordial ways that let molecules move from one place to another is called simple diffusion, and although it could result pretty obvious, it is interesting to elucidate from which parameters depends and to what extent they can describe important aspects of our physiology.
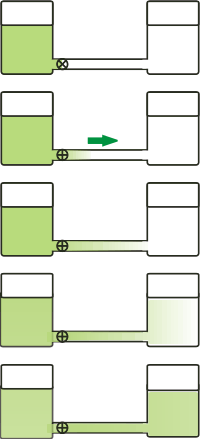
Yes, obvious, that's what I said: imagine a pair of water-filled containers up to the same level and connected by a horizontal tube with a closed stopcock. Now let say we dye the water in one of the containers with a green ink: quintillions of ink molecules enter in the solution… and our experiment is about to begin. |
As soon as we open the stopcock, we notice that the connecting tube is stained progressively, revealing a movement from the dyed water-filled tank to the other
This movement is called diffusive flux, and is triggered by the thermal agitation of both the solute molecules (the one from the dyed water) such as those of the solvent (clean water)
While thermal agitation is a chaotic movement, its result -besides being obvious- is quantifiable and predictable.
The migration of solution molecules becomes evident (in our experiment) because the pure water dyes, and the dyed water fades.
No movement is observed when the coloration of both containers is the same, from where we deduct that the phenomenon is extinguished when the concentration in both containers is the same.
But before closing the case, we could ask ourselves how to define this movement, and from which variables it depends.
|
|
|
|
|
Definition: we call diffusive flux (which we will symbolize with φ) to the quotient between the total mass of solute molecules passing through a section of the connection tube and the time interval in which it crosses:
φ = m / Δt
As the diffusive flux is the result of a chaotic and random phenomenon in which the molecules come and go in all directions, it must be remembered that the definition refers to the net movement, in a single sense, from where the solute is more concentrated to where it is more diluted... as the arrow indicates.
The more common units used to measure the diffusive flux are:
[φ] = moles / s
And now let's go to the second question: What makes the speed of this phenomenon to change? Is not hard: |
|
|
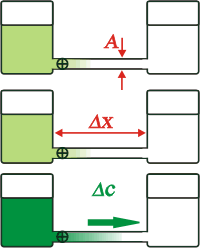
The larger the area - the section of the tube through which solute molecules travel - the greater the flow.
The longer the tube connecting the containers, the less the flow.
The higher the solute concentration in the dyed water container... or rather, the greater the difference in solute concentration along the tube, the greater the flow.
It has bee experimentally confirmed that each of these parameters has a simple proportionality to the flow, but one is missing.
|
|
|
|
|
Each molecule of solute will have its own mobility, that is characteristic to it in each solvent. Some (usually the smaller ones) will have more mobility and others (generally the more bulky ones) will move less. This property is called diffusion coefficient and is symbolized D.
All this can be summed up in a single equation, called Fick's Law: |
Watch it!
Look carefully, these two letters are different:
φ, Φ;
The first is lowercase; The second, uppercase.
|
|
The minus sign is because the flow has opposite direction to the concentration difference, since the net movement occurs from the place of highest concentration to the lowest concentration.
Many times, another magnitude results more useful: flow density (simbolized Φ). We could define it as the amount of flow per unit area. Just by dividng both members by the are, we get: |
|
And its units will be:
[Φ] = moles/m2s
|
Note: There is no agreement on textbooks to designate neither the name or the symbol of diffusive flux, or flow density, so -while working whith them- the concept is usually interpreted by the units of magnitude: if it is diving a unit of area, we are talking about flow density; else, it is about difusive flux.
|
|
|
Curious Facts:
|
|
|
- The simple diffusion can take place through a porous membrane, as long as the pores do not place restrictions to the passage -for one side and for another- of the solute molecule. If this is the case (which is one of the most interesting in physiology) the pore length is none other than Δx, and also is equal to the thickness of the membrane; the concentration difference, then, is the one that exists between both sides of the membrane, And in general the area is the sum of the areas of all pores (which are usually all the same) that we find in the little membrane that we have under observation.
- In order to predict the net motion of a molecule, only the concentration difference of that molecule is of interest. As for the movement of diffusive flux, each group or type of molecule behaves as if they were the only ones in the universe. Understanding the latter is essential to understanding the mechanism of nerve impulse.
|
|

|
Captious questions: |
|
- In what units should the diffusion coefficients be measured?
- Would the dissolved gases in the body fluids (O2, CO2, N2, etc.)...diffuse freely through the plasma membranes?
|
|
| |
|
|
| Some rights reserved. Reproduction permitted if quoting the source. Last updated on Dec-16. Translated by Esteban Djeordjian. Buenos Aires, Argentina. |
|
|

|
| | |
|
|
![]()
![]()
![]()