|
NO ME SALEN
(APUNTES TEÓRICOS DE BIOFÍSICA DEL CBC)
EVOLUCIONES REVERSIBLES DE GASES IDEALES
|
|

Both W and L are ways to symbolize work |
|
| |
Reversible evolution of ideal gases
An evolution of a gas between two different states (different volume, different pressure and different temperature) is usually represented by pressure-volume graphs like the ones below. There are no restrictions for evolutions that occur in real life, and in most of the cases they are so chaotic that attempting a graphical representation of a real evolution could be considered utopian.
However, an extremely slow and ordered evolution would allow us to know at every moment all the thermodynamic variables of the gas. This is called reversible evolution and its main property is that every gas intermediate state between any two states is completely defined (its known).
The information that these ideal evolutions give us is valid and allows to establish precise limits to the real transformations that occur in the universe. Also, it is very common (and useful) to use them for approximations for calculations and inferences. That's why we study them.
In this table I put the main evolutions, followed by the equations used to calculate the energy implied in the First Law of the Thermodynamics,
Q = ΔU + W
|
| |
|
|
|
|
|
|
|
| |
isobaric |
isochoric |
isothermal |
adiabatic |
cycle |
any |
| Q |
cp n ΔT |
cv n ΔT |
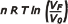 |
0 |
 |
|
| ΔU |
cv n ΔT |
cv n ΔT |
0 |
cv n ΔT |
0 |
cv n ΔT |
| W |
p ΔV |
0 |
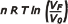 |
– cv n ΔT |
 |
|
Where cV and cP, are the specific molar heat at constant volume
and the specific molar heat at constant pressure, respectively;
for ideal monatomic gases cV = 1,5 R and cP = 2,5 R, and for diatomics cV = 2,5 R and cP = 3,5 R,
and where R is the ideal gas constant:
R = 8,314 J/mol K = 0,08207 l atm/mol K = 1,987207 cal/K mol |
|
Without much detail, the basic guidelines are:
- the heat, Q, is calculated with the same criterion as in calorimetry.
- The work, W, is calculated by performing the integral: W = ∫ p dV; from this arises:
n R T ln (VF /V0).
- The internal energy of a gas, ΔU, depends (basically) on the temperature, and it is also a state function (in two articles, I’ll go deeper on this). In the case of an ideal gas, the internal energy depends exclusively on the temperature.
- It is always useful to have the state equation of the ideal gases, P V = n R T, which can help you in case of a hurry. For example...
In an isobaric evolution, cp n ΔT = cp P ΔV / R
In an isochronous evolution, cv n ΔT = cv V ΔP / R
In an isothermal evolution, n R T ln (VF /V0) = n R T ln (p0 /pF )
|
|
|
|
|
|
CURIOUS FACTS |
|
|
- When Wilhelm Ostwald (1853-1932), Nobel Prize for Chemistry in 1909, introduced the term mol in 1900, he defined it on the basis of a mass proportional to the relative masses of the elemental entities of substances, which brought the usual problem of the interpretation of the mol as a unit of mass.
Later, after some advances in the atomic-molecular theory, a mol was identified as a fixed number of atoms, molecules, ions, and so on. That is, the Avogrado number of elementary entities.
It is clear, then, that the mole was born to establish any number of entities.
- Air is a mixture of fundamental -and mostly diatomic- gases, and behaves almost like an ideal gas
|
|
|
 |
CAPTIOUS QUESTIONS |
|
|
- What volume would be occupied by one mol of an ideal gas at 1 atm pressure and at 0 ° C of temperature?
- It is said that a reversible or definite evolution must occur slowly ... but what does it mean slowly? Slower than what?
|
|
|
| |
|
|
NOTE: Don't use the table of this article as a summary... brings bad luck. A few years ago I prepared it for a student who used it to study, and got a D-. In the following course he lent it to a partner and he got an E. He noticed the bad luck that the summary had brought them, and he threw it out the window. A bus driver got distracted looking a the sheet of paper falling dow, and he hit a traffic light: 3 dead and 15 wounded. Just don't use it!
If you could invest two hours making your own summary, you would understand and learn much more, and you could write down a lot more thing that could be more useful for the exam
|
|
|
 |
| |
| |
|
|
| Some rights reserved. Reproduction permitted if quoting the source. Last updated on Feb-17.Translated by Esteban Djeordjian. Buenos Aires, Argentina. |
|
|
 |
| | |
|
|